|
Human Endocrine System
Endocrinology |
||||||||||||||||
| Endocrine Diseases | ||
| Assessment of a patient by a physician. | ||
| What are various symptoms, signs, statements, questions, issues, and histories that should raise suspicion of a medical emergency? | Answer | |
| How should you do a quick assessment, diagnosis, and treatment of a person reported as an endocrinological medical emergency? | Answer | |
| Medical emergency situation: What questions will you ask? | Answer | |
| Medical nonemergency situation: What questions will you ask? | Answer | |
| How will you further proceed in this situation? | Answer | |
| Anatomy of the Endocrine System | ||
| How many endocrine system organs are there in the human body? | Answer | |
| Can you name the endocrine system organs in the human body? | Answer | |
| Functions of the Endocrine System | ||
| What does the endocrine system do? | Answer | |
| What problems can happen with your endocrine system? | Answer | |
| Physiology of the Endocrine System | ||
| How do hormones in the endocrine system move around the body? | Answer | |
| Drugs Used to Treat Endocrine Disorders | ||
| Drugs Used to Treat Endocrine Disorders | Answer | |
| Practice Quiz: Endocrine System Anatomy and Physiology | ||
| Which gland in the brain secretes antidiuretic hormone (ADH)? | Answer | |
| How do hormones in the endocrine system move around the body? | Answer | |
| Where are the adrenal glands located? | Answer | |
| Which of these is NOT an endocrine function of the pancreas? | Answer | |
| What type of hormone is testosterone? | Answer | |
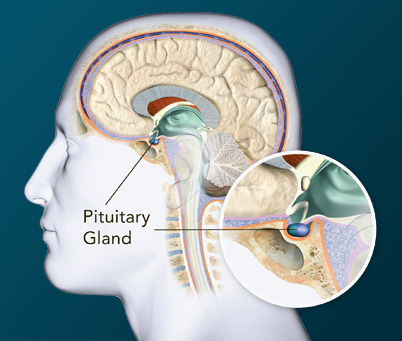
| Where is the pituitary gland located? | Answer | |
| Which reflex is adrenaline most often associated with? | Answer | |
| What is the name given to absolute insulin deficiency? | Answer | |
| Which hormone increases blood glucose levels? | Answer | |
| Where are the parathyroid glands located? | Answer | |
| Endocrine Diseases | ||
| What are various examples? | Answer | |
| Symptoms and sign | ||
| What are various symptoms, signs, statements, questions, issues, and histories that should raise suspicion of a medical emergency? | Answer | |
| What can be symptoms or complaints relevant to the human endocrine system? | Answer | |
| Emergencies | ||
| What are endocrinological medical emergencies? | Answer | |
| Tests and Procedures | ||
| What is the range of normal human biochemistry laboratory values? | Answer | |
| What ranges of normal human biochemistry values do you follow? | Answer | |
| Diagnosis and Treatment | ||
| What are various endocrinologic disabilities? | Answer | |
| Endocrinology consultation | ||
| Endocrinologist | ||
| What is the most common condition relevant to endocrinology? | Answer | |
| What is the most common condition relevant to endocrinology? | Answer | |
| __________ | Answer | |
| Prevention | ||
| How can you prevent endocrine problems? | Answer | |
| What is endocrinology? | Answer | |
What are various symptoms, signs, statements, questions, issues, and histories that should raise suspicion of a medical emergency?
Endocrine emergencies Alcoholic Ketoacidosis
|
What is alcoholic ketoacidosis? Cells need glucose (sugar) and insulin to function properly. Glucose comes from the food you eat, and insulin is produced by the pancreas. When you drink alcohol, your pancreas may stop producing insulin for a short time. Without insulin, your cells won’t be able to use the glucose you consume for energy. To get the energy you need, your body will start to burn fat. When your body burns fat for energy, byproducts known as ketone bodies are produced. If your body is not producing insulin, ketone bodies will begin to build up in your bloodstream. This buildup of ketones can produce a life-threatening condition known as ketoacidosis. Ketoacidosis, or metabolic acidosis, occurs when you ingest something that is metabolized or turned into an acid. This condition has a number of causes, including: large doses of aspirin shock kidney disease abnormal metabolism In addition to general ketoacidosis, there are several specific types. These types include: alcoholic ketoacidosis, which is caused by excessive consumption of alcohol diabetic ketoacidosis (DKA), which mostly develops in people with type 1 diabetes starvation ketoacidosis, which occurs most often in women who are pregnant, in their third trimester, and experiencing excessive vomiting Each of these situations increases the amount of acid in the system. They can also reduce the amount of insulin your body produces, leading to the breakdown of fat cells and the production of ketones. What causes alcoholic ketoacidosis? Alcoholic ketoacidosis can develop when you drink excessive amounts of alcohol for a long period of time. Excessive alcohol consumption often causes malnourishment (not enough nutrients for the body to function well). People who drink large quantities of alcohol may not eat regularly. They may also vomit as a result of drinking too much. Not eating enough or vomiting can lead to periods of starvation. This further reduces the body’s insulin production. If a person is already malnourished due to alcoholism, they may develop alcoholic ketoacidosis. This can occur as soon as one day after a drinking binge, depending on nutritional status, overall health status, and the amount of alcohol consumed. What are the symptoms of alcoholic ketoacidosis? The symptoms of alcoholic ketoacidosis will vary based on how much alcohol you have consumed. Symptoms will also depend on the amount of ketones in your bloodstream. Common symptoms of alcoholic ketoacidosis include: abdominal pain agitation and confusion decreased alertness or coma fatigue slow movement irregular, deep, and rapid breathing (Kussmaul’s sign) loss of appetite nausea and vomiting symptoms of dehydration, such as dizziness (vertigo), lightheadedness, and thirst If you develop any of these symptoms, seek emergency medical attention. Alcoholic ketoacidosis is a life-threatening illness. Someone with alcoholic ketoacidosis may also have other conditions that are associated with alcohol abuse. These may include: pancreatitis liver disease kidney disease ulcers ethylene glycol poisoning These conditions have to be ruled out before a medical professional can diagnose you with alcoholic ketoacidosis. How is alcoholic ketoacidosis diagnosed? If you have symptoms of alcoholic ketoacidosis, your doctor will perform a physical examination. They will also ask about your health history and alcohol consumption. If your doctor suspects that you’ve developed this condition, they may order additional tests to rule out other possible conditions. After these test results are in, they can confirm the diagnosis. Tests may include the following: amylase and lipase tests, to monitor the functioning of your pancreas and check for pancreatitis arterial blood gas test, to measure your blood’s oxygen levels and acid/base balance anion gap calculation, which measures sodium and potassium levels blood alcohol test blood chemistry panel (CHEM-20), to get a comprehensive look at your metabolism and how well it’s functioning blood glucose test blood urea nitrogen (BUN) and creatinine tests, to determine how well your kidneys are functioning serum lactate test, to determine levels of lactate in the blood (high lactate levels can be a sign of lactic acidosis, a condition that usually indicates that the body’s cells and tissues are not receiving enough oxygen) urine test for ketones If your blood glucose level is elevated, your doctor may also perform a hemoglobin A1C (HgA1C) test. This test will provide information about your sugar levels to help determine whether you have diabetes. If you have diabetes, you may need additional treatment. How is alcoholic ketoacidosis treated? Treatment for alcoholic ketoacidosis is typically administered in the emergency room. Your doctor will monitor your vital signs, including your heart rate, blood pressure, and breathing. They will also give you fluids intravenously. You may receive vitamins and nutrients to help treat malnutrition, including: thiamine potassium phosphorus magnesium Your doctor may also admit you to the intensive care unit (ICU) if you require ongoing care. The length of your hospital stay depends on the severity of the alcoholic ketoacidosis. It also depends on how long it takes to get your body regulated and out of danger. If you have any additional complications during treatment, this will also affect the length of your hospital stay. What are the complications of alcoholic ketoacidosis? One complication of alcoholic ketoacidosis is alcohol withdrawal. Your doctor and other medical professionals will watch you for symptoms of withdrawal. If you have severe symptoms, they may give you medication. Alcoholic ketoacidosis may lead to gastrointestinal bleeding. Other complications may include: psychosis coma pancreatitis pneumonia encephalopathy (a brain disease that can cause memory loss, personality changes, and muscle twitching, though this is uncommon) What is the long-term outlook for alcoholic ketoacidosis? If you are diagnosed with alcoholic ketoacidosis, your recovery will depend on a number of factors. Seeking help as soon as symptoms arise reduces your chances of serious complications. Treatment for alcohol addiction is also necessary to prevent a relapse of alcoholic ketoacidosis. Your prognosis will be impacted by the severity of your alcohol use and whether or not you have liver disease. Prolonged used of alcohol can result in cirrhosis, or permanent scarring of the liver. Cirrhosis of the liver can cause exhaustion, leg swelling, and nausea. It will have a negative effect on your overall prognosis. How can I prevent alcoholic ketoacidosis? You can prevent alcoholic ketoacidosis by limiting your alcohol intake. If you are addicted to alcohol, seek professional help. You can learn how to reduce your alcohol intake or eliminate it altogether. Joining a local chapter of Alcoholics Anonymous may provide you with the support you need to cope. You should also follow all of your doctor’s recommendations to ensure proper nutrition and recovery. |
Type 1 diabetes mellitus with ketoacidosis without coma
|
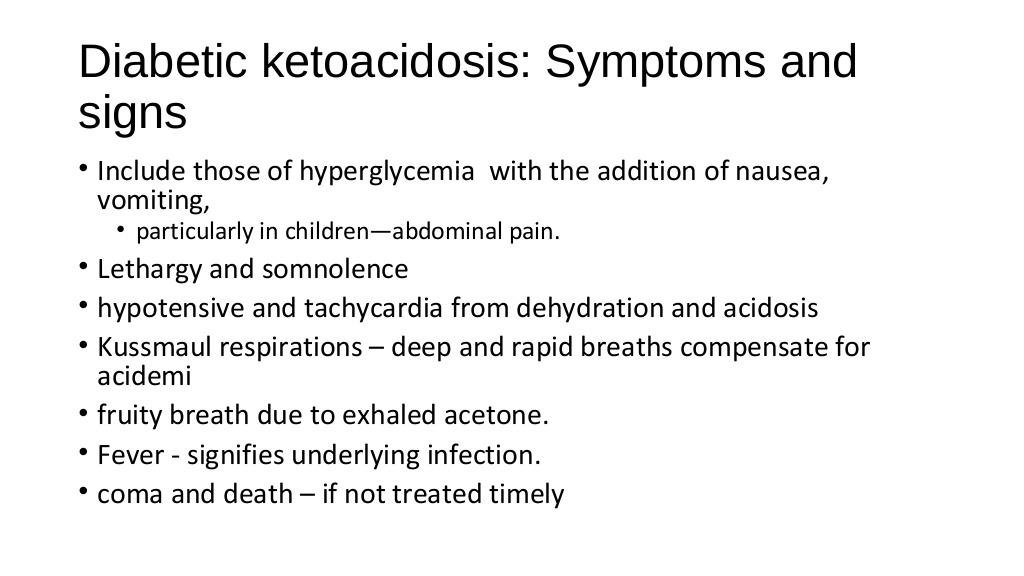
What is diabetic ketoacidosis? Diabetic ketoacidosis, also referred to as simply ketoacidosis or DKA, is a serious and even life-threatening complication of type 1 diabetes. DKA is rare in people with type 2 diabetes. DKA is caused when insulin levels are low and not enough glucose can get into the body's cells. Without glucose for energy, the body starts to burn fat for energy. Ketones are products that are created when the body burns fat. The buildup of ketones causes the blood to become more acidic. The high levels of blood glucose in DKA cause the kidneys to excrete glucose and water, leading to dehydration and imbalances in body electrolyte levels. Diabetic ketoacidosis most commonly develops either due to an interruption in insulin treatment or a severe illness, including the flu. What are the symptoms and signs of diabetic ketoacidosis? The development of DKA is usually a slow process. However, if vomiting develops, the symptoms can progress more rapidly due to the more rapid loss of body fluid. Early signs and symptoms of DKA include: 1. Thirst, which arises due to dehydration 2.Excessive urination, which occurs because the kidneys try to rid the body of excess glucose, and water is excreted along with the glucose 3.High blood glucose (sugar?) levels 4.The presence of ketones in the urine Other signs and symptoms of ketoacidosis occur as the condition progresses: These include: 1. Fatigue, which can be severe 2. Nausea and/or vomiting 3. Abdominal pain 4.Flushing of the skin 5. Dry skin 6.Fruity odor to the breath, caused by ketones 7.Difficulty breathing 8.Mental status changes, including confusion or problems with concentration What should you do if you think you may have, or someone you know may diabetic ketoacidosis? You should test your urine for ketones if you suspect you have early symptoms or warning signs of ketoacidosis. Call your health-care professional if your urine shows high levels of ketones. High levels of ketones and high blood sugar (glucose) levels, particularly if vomiting is present, can be warning signs for the development of DKA. Always seek medical assistance in this situation. Contact your doctor immediately or go to a hospital emergency department. DKA requires treatment in the hospital with intravenous fluid and electrolyte replacement and insulin. |
Diabetic Coma and Type 2 Diabetes
|
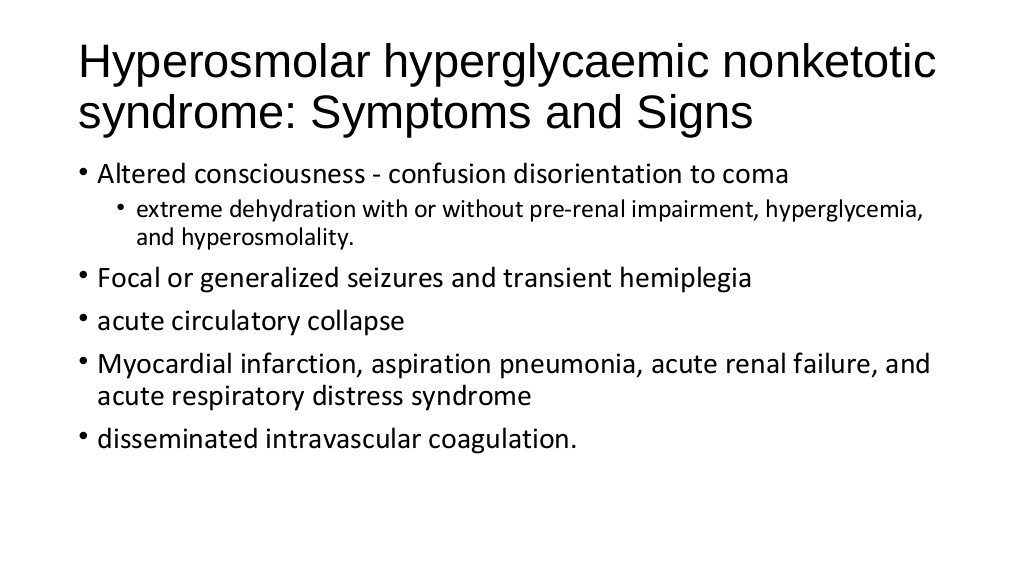
What is hyperosmolar hyperglycemic syndrome (HHS)? Hyperosmolar hyperglycemic syndrome (HHS) is a serious complication of diabetes mellitus. HHS occurs when a person’s blood glucose (sugar) levels are too high for a long period, leading to severe dehydration (extreme thirst) and confusion. Hyperosmolar hyperglycemic syndrome is also known by many other names, including: •Diabetic HHS. •Diabetic hyperosmolar syndrome. •Hyperglycemic hyperosmolar nonketotic coma (HHNK). •Hyperosmolar coma. •Hyperosmolar hyperglycemic nonketotic syndrome (HHNS). •Hyperosmolar hyperglycemic state. •Nonketotic hyperosmolar syndrome (NKHS). Who is affected by hyperosmolar hyperglycemic syndrome (HHS)? HHS most often affects people who have type 2 diabetes who are: •Older (usually in their 60s or 70s). •African-American, Native American or Hispanic. •Affected by other health issues, such as infection, illness or heart conditions. HHS can be fatal if it’s not treated. Rarely, HHS can affect children and young adults who have type 1 or type 2 diabetes, especially if they are obese. Very rarely, people who have not yet been diagnosed with diabetes can develop HHS. How common is hyperosmolar hyperglycemic syndrome (HHS)? HHS is less common than other major complications associated with diabetes. HHS accounts for less than 1% of hospital admissions for people with diabetes. What causes hyperosmolar hyperglycemic syndrome (HHS)? People who have diabetes have too much glucose (sugar) in their blood. The glucose builds up because their bodies either don’t make enough insulin, or have trouble using the insulin that they do make. (Insulin is a naturally occurring hormone, produced by the beta cells of the pancreas, which helps the body use sugar for energy.) HHS occurs when the blood sugar of a person with diabetes becomes too high (hyperglycemia) for a long time. The extra sugar is passed into the urine, which causes the person to urinate frequently. As a result, he or she loses a lot of fluid, which can lead to severe dehydration (extreme thirst). HHS usually develops in people who do not have their type 2 diabetes under control and they: •Have an illness or infection, such as pneumonia or a urinary tract infection. •Stop taking medication to manage their diabetes. •Have a heart attack or stroke. •Take certain medications—such as steroids or diuretics—that can cause the syndrome. What are the symptoms of hyperosmolar hyperglycemic syndrome (HHS)? Symptoms of HHS usually come on slowly, and can take days or weeks to develop. Symptoms include: •High blood sugar level (over 600 mg/dL). •Confusion, hallucinations, drowsiness or passing out. •Dry mouth and extreme thirst that may eventually get better. •Frequent urination. •Fever over 100.4 degrees Fahrenheit. •Blurred vision or loss of vision. •Weakness or paralysis that may be worse on one side of the body. How is hyperosmolar hyperglycemic syndrome (HHS) diagnosed? You should seek medical attention right away if you are diabetic and you have these symptoms: •Extreme thirst. •Frequent urination. •Confusion, or a change in your mental state. •Changes in your vision. Your doctor will examine you, ask about your symptoms, and order a blood test to check your blood sugar level. A very high blood sugar level (over 600 mg/dL) with low ketone levels (acids in blood and urine) will help the doctor make a diagnosis of HHS. How is hyperosmolar hyperglycemic syndrome (HHS) treated? To treat HHS, your doctor will give you intravenous (IV) medications. These include: •Fluids to hydrate you. •Electrolytes (such as potassium) to balance the minerals in your body. •Insulin to control your blood sugar levels. Your doctor will also treat any underlying conditions or infections that may have caused the HHS. You will usually stay in the hospital so that your healthcare team can watch you closely for any complications. What are the side effects of the treatment for hyperosmolar hyperglycemic syndrome (HHS)? The IV electrolytes or fluids used to treat dehydration do not have side effects. Side effects from insulin include: •Hypoglycemia (low blood sugar). •Swelling of the arms and legs. •Weight gain. Your doctor will treat you for hypoglycemia if your blood sugar gets too low while you are in the hospital. What are the complications associated with hyperosmolar hyperglycemic syndrome (HHS)? HHS is a very serious medical condition. If it is not treated, it can lead to: •Seizures. •Coma. •Swelling of the brain. •Organ failure. •Death. What can you do to relieve symptoms of hyperosmolar hyperglycemic syndrome (HHS)? If you have symptoms of HHS, you should drink plenty of water and call 911 or go to the emergency room immediately. You will receive an IV with fluids and insulin to relieve your symptoms. Can hyperosmolar hyperglycemic syndrome (HHS) be prevented? The best way to prevent HHS is by following a healthy lifestyle and managing your diabetes. You should: •Check your blood sugar frequently to make sure you’re staying within your target range. •Take your insulin and other diabetes medications as directed by your doctor. •Follow a healthy diet. •Never drink alcohol on an empty stomach. •Get more rest and check your blood sugar more often when you are sick. •Know the symptoms of HHS and get help right away if you have any of them. What is the prognosis (outlook) for patients who have hyperosmolar hyperglycemic syndrome (HHS)? The outlook for patients who have HHS largely depends on the person’s age, general health and how severe the disease is. Up to 20% of people who have HHS die from the condition. If you’ve had HHS, you will need to work closely with your doctor once you are home from the hospital. You can reduce your risk of developing HHS again by controlling your diabetes and managing your diet and lifestyle. |
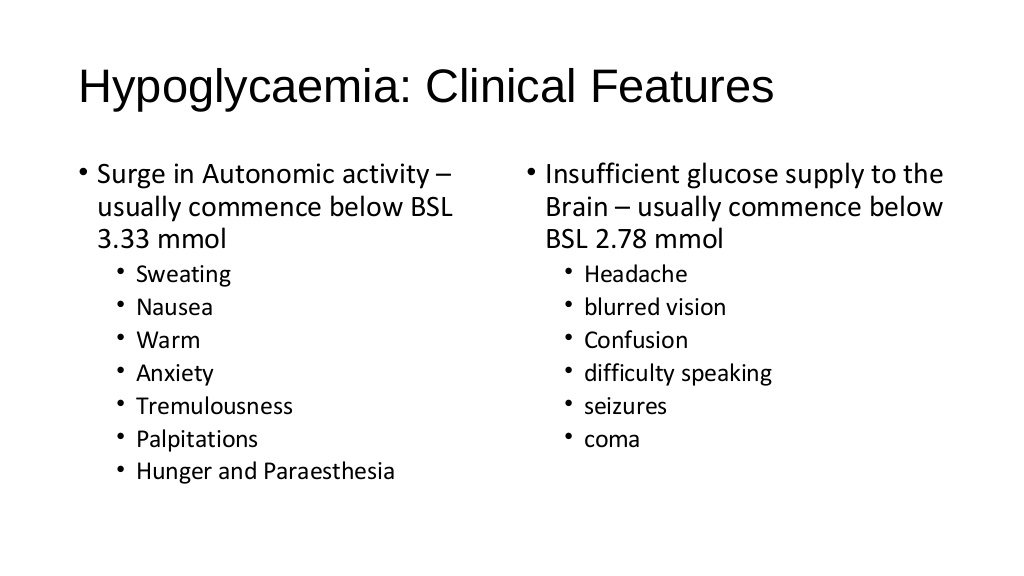
Hypoglycemia
|
What is it? Hypoglycemia needs immediate treatment when blood sugar levels are low. For many people, a fasting blood sugar of 70 milligrams per deciliter (mg/dL), or 3.9 millimoles per liter (mmol/L), or below should serve as an alert for hypoglycemia. But your numbers might be different. Ask your doctor. Hypoglycemia is characterized by a reduction in plasma glucose concentration to a level that may induce symptoms or signs such as altered mental status and/or sympathetic nervous system stimulation. This condition typically arises from abnormalities in the mechanisms involved in glucose homeostasis. The most common cause of hypoglycemia in patients with diabetes is injecting a shot of insulin and skipping a meal or overdosing insulin. Treatment involves quickly getting your blood sugar back to normal either with high-sugar foods or drinks or with medications. Long-term treatment requires identifying and treating the cause of hypoglycemia. What causes it? Possible causes, without diabetes Hypoglycemia in people without diabetes is much less common. Causes can include the following: •Medications. Taking someone else's oral diabetes medication accidentally is a possible cause of hypoglycemia. Other medications can cause hypoglycemia, especially in children or in people with kidney failure. One example is quinine (Qualaquin), used to treat malaria. •Excessive alcohol drinking. Drinking heavily without eating can block your liver from releasing stored glucose into your bloodstream, causing hypoglycemia. •Some critical illnesses. Severe liver illnesses such as severe hepatitis or cirrhosis can cause hypoglycemia. Kidney disorders, which can keep your body from properly excreting medications, can affect glucose levels due to a buildup of those medications. Long-term starvation, as can occur in the eating disorder anorexia nervosa, can result in too little of substances your body needs to create glucose. •Insulin overproduction. A rare tumor of the pancreas (insulinoma) can cause you to produce too much insulin, resulting in hypoglycemia. Other tumors also can result in too much production of insulin-like substances. Enlargement of cells of the pancreas that produce insulin can result in excessive insulin release, causing hypoglycemia. •Hormone deficiencies. Certain adrenal gland and pituitary tumor disorders can result in a deficiency of key hormones that regulate glucose production. Children can have hypoglycemia if they have too little growth hormone. Hypoglycemia after meals Hypoglycemia usually occurs when you haven't eaten, but not always. Sometimes hypoglycemia symptoms occur after certain meals high in sugar because your body produces more insulin than you need. This type of hypoglycemia, called reactive hypoglycemia or postprandial hypoglycemia, can occur in people who have had stomach bypass surgery. It can also occur in people who haven't had this surgery. Complications Untreated hypoglycemia can lead to: •Seizure •Loss of consciousness •Death Hypoglycemia can also contribute to the following: •Dizziness and weakness •Falls •Injuries •Motor vehicle accidents •Greater risk of dementia in older adults What is a low blood sugar emergency? There are different levels of low blood sugar: mild, moderate, or severe. If your blood sugar drops low enough that you need help to recover, it is considered to be a low blood sugar emergency, or severe hypoglycemia. Mild or moderate low blood sugar is common for people with type 1 diabetes, and can occur in people with type 2 diabetes who are using insulin. If not treated, mild or moderate low blood sugar can progress and become severe, requiring the help of someone else to recover. Prevention How can you prevent hypoglycemia? If you have diabetes Follow the diabetes management plan you and your doctor have developed. If you're taking new medications, changing your eating or medication schedules, or adding new exercise, talk to your doctor about how these changes might affect your diabetes management and your risk of low blood sugar. A continuous glucose monitor (CGM) is an option for some people, particularly those with hypoglycemia unawareness. A CGM has a tiny wire that's inserted under the skin that can send blood glucose readings to a receiver. If blood sugar levels are dropping too low, some models of CGM will alert you with an alarm. Some insulin pumps are now integrated with CGMs and can shut off insulin delivery when blood sugar levels are dropping too quickly to help prevent hypoglycemia. Be sure to always have a fast-acting carbohydrate with you, such as juice or glucose tablets so that you can treat a falling blood sugar level before it dips dangerously low. If you don't have diabetes For recurring episodes of hypoglycemia, eating frequent small meals throughout the day is a stopgap measure to help prevent your blood sugar levels from getting too low. However, this approach isn't advised as a long-term strategy. Work with your doctor to identify and treat the cause of hypoglycemia. If you have diabetes, ways you can prevent hypoglycemia include: •Follow your meal plan. •Eat at least three evenly spaced meals each day with between-meal snacks as prescribed. •Plan your meals no more than four to five hours apart. •Exercise 30 minutes to one hour after meals. Check your sugars before and after exercise, and discuss with your doctor what types of changes can be made. •Double-check your insulin and dose of diabetes medicine before taking it. •If you drink alcohol, be moderate and monitor your blood sugar levels. •Know when your medicine is at its peak level. •Test your blood sugar as often as directed by your doctor. •Carry an identification bracelet that says you have diabetes. |
Thyroid storm
|
What is thyroid storm? Thyroid storm is a life-threatening health condition that is associated with untreated or undertreated hyperthyroidism. During thyroid storm, an individual’s heart rate, blood pressure, and body temperature can soar to dangerously high levels. Without prompt, aggressive treatment, thyroid storm is often fatal. The thyroid is a small, butterfly-shaped gland located in the middle of your lower neck. The two essential thyroid hormones produced by the thyroid are triiodothyronine (T3) and thyroxine (T4). These control the rate at which every cell in your body works (your metabolism). If you have hyperthyroidism, your thyroid is producing too much of these two hormones. This causes all of your cells to work too quickly. For example, your respiration rate and heart rate will be higher than they normally would be. You may even speak far more quickly than you usually do. Causes of thyroid storm Thyroid storm is rare. It develops in people who have hyperthyroidism but aren’t receiving appropriate treatment. This condition is marked by the extreme overproduction of the two hormones produced by the thyroid gland. Not all people with hyperthyroidism will develop thyroid storm. Causes of this condition include: severe undertreated hyperthyroidism untreated overactive thyroid gland infection associated with hyperthyroidism People with hyperthyroidism may develop thyroid storm after experiencing one of the following: trauma surgery severe emotional distress stroke diabetic ketoacidosis congestive heart failure pulmonary embolism Symptoms of thyroid storm Symptoms of thyroid storm are similar to those of hyperthyroidism, but they are more sudden, severe, and extreme. This is why people with thyroid storm might not be able to seek care on their own. Common symptoms include: racing heart rate (tachycardia) that exceeds 140 beats per minute, and atrial fibrillation high fever persistent sweating shaking agitation restlessness confusion diarrhea unconsciousness Diagnosing thyroid storm Individuals with hyperthyroidism who experience any symptoms of thyroid storm are typically admitted to an emergency room. If you suspect you or someone else has thyroid storm symptoms, call 911 immediately. People with thyroid storm generally exhibit an increased heart rate, as well as a high top blood pressure number (systolic blood pressure). A doctor will measure your thyroid hormone levels with a blood test. Thyroid stimulating hormone (TSH) levels tend to be low in hyperthyroidism and thyroid storm. According to the American Association for Clinical Chemistry (AACC), normal values for TSH range from 0.4 to 4 milli–international units per liter (mIU/L). T3 and T4 hormones are higher than normal in people with thyroid storm. Treating this condition Thyroid storm develops abruptly and affects all the systems of your body. Treatment will begin as soon as thyroid storm is suspected — usually before lab results are ready. Antithyroid medication like propylthiouracil (also called PTU) or methimazole (Tapazole) will be given to reduce the production of these hormones by the thyroid. Hyperthyroidism requires ongoing care. People with hyperthyroidism may be treated with radioactive iodine, which destroys the thyroid, or a course of drugs to suppress thyroid function temporarily. Pregnant women who have hyperthyroidism can’t be treated with radioactive iodine because it would harm the unborn child. In those cases, the woman’s thyroid would be removed surgically. People experiencing thyroid storm should avoid taking iodine in lieu of medical treatment, as this can worsen the condition. If your thyroid is destroyed by radioactive iodine treatment or removed surgically, you will need to take synthetic thyroid hormone for the rest of your life. Long-term outlook Thyroid storm requires immediate, aggressive emergency medical attention. When left untreated, thyroid storm can cause congestive heart failure or fluid-filled lungs. The mortality rateTrusted Source for people with untreated thyroid storm is estimated to be 75 percent. The chances of surviving thyroid storm increase if you quickly seek medical care. Related complications may be lessened once your thyroid hormone levels are returned to the normal range (known as euthyroid). Preventing thyroid storm The most effective way to prevent the onset of thyroid storm is to keep up with your thyroid health plan. Take your medications as instructed. Keep all appointments with your doctor and follow through with blood work orders as needed. |
Acute adrenal insufficiency
|
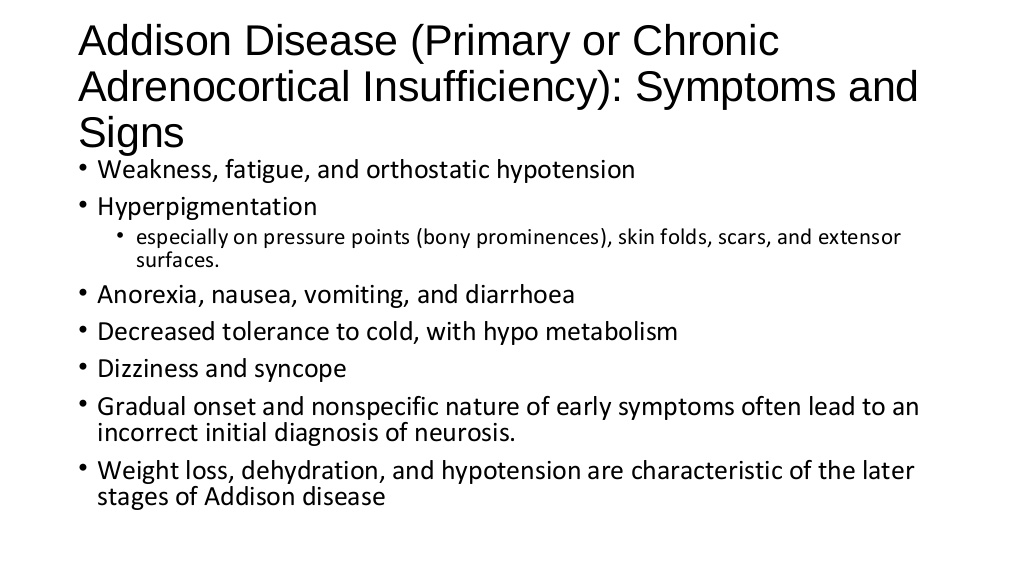
Definition: Acute adrenal crisis is a life-threatening state caused by insufficient levels of cortisol, which is a hormone produced and released by the adrenal gland. Alternative Names: Adrenal crisis; Addisonian crisis; Acute adrenal insufficiency Causes, incidence, and risk factors: The two adrenal glands are located on top of the kidneys. They consist of the outer portion, called the cortex, and the inner portion, called the medulla. The cortex produces three types of hormones, all of which are called corticosteroids. Cortisol is a glucocortoid, a corticosteroid that maintains glucose (blood sugar) regulation, suppresses the immune response, and is released as part of the body's response to stress. Cortisol production is regulated by a small gland just below the brain called the pituitary gland. Cortisol is essential for life. Acute adrenal crisis is a medical emergency caused by a lack of cortisol. Patients may experience lightheadedness or dizziness, weakness, sweating, abdominal pain, nausea and vomiting, or even loss of consciousness. Adrenal crisis occurs if the adrenal gland is deteriorating (Addison's disease, primary adrenal insufficiency), if there is pituitary gland injury (secondary adrenal insufficiency), or if adrenal insufficiency is not adequately treated. Risk factors for adrenal crisis include physical stress such as infection, dehydration, trauma, or surgery, adrenal gland or pituitary gland injury, and ending treatment with steroids such as prednisone or hydrocortisone too early. Symptoms: •Headache •Profound weakness •Fatigue •Slow, sluggish movement •Nausea •Vomiting •Low blood pressure •Dehydration •High fever •Shaking chills •Confusion or coma •Darkening of the skin •Rapid heart rate •Joint pain •Abdominal pain •Unintentional weight loss •Rapid respiratory rate (see tachypnea) •Unusual and excessive sweating on face and/or palms •Skin rash or lesions may be present •Flank pain •Loss of appetite Signs and tests: •An ACTH (cortrosyn) stimulation test shows low cortisol. •The baseline cortisol level is low. •Fasting blood sugar may be low. •Serum potassium is elevated ( usually primary adrenal insufficiency). •Serum sodium is decreased (usually primary adrenal insufficiency). Treatment: In adrenal crisis, an intravenous or intramuscular injection of hydrocortisone (an injectable corticosteroid) must be given immediately. Supportive treatment of low blood pressure with intravenous fluids is usually necessary. Hospitalization is required for adequate treatment and monitoring. If infection is the cause of the crisis, antibiotic therapy may be needed. Expectations (prognosis): Death may occur due to overwhelming shock if early treatment is not provided. Complications: •shock •coma •seizures Calling your health care provider: Call your health care provider if you have Addison's disease and are unable to retain usual medications because of vomiting.Go to the emergency room or call the local emergency number if symptoms of acute adrenal crisis develop. Prevention: People who have Addison's disease should be taught to recognize signs of potential stress that may cause an acute adrenal crisis. Most people with Addison's disease are taught to give themselves an emergency injection of hydrocortisone or increase their dose of oral prednisone in times of stress. It is important for the individual with Addison's disease to always carry a medical identification card that states the type of medication and the proper dose needed in case of an emergency. Never omit medication. If unable to retain medication due to vomiting, notify the health care provider. |
Pituitary Gland Failure (Pituitary Apoplexy)
|
What is Pituitary Apoplexy? Pituitary apoplexy occurs when a pituitary adenoma either spontaneously hemorrhages or grows in such a way as to compress and cut off its own blood supply, resulting in tumor cell death, bleeding, and acute swelling. Symptoms Pituitary Gland Failure (Pituitary Apoplexy) Sudden And Severe Headache Double Vision Or Other Visual Impairments Paralysis of the eye muscles, causing double vision (ophthalmoplegia) or problems opening an eyelid. Loss of peripheral vision or loss of all vision in one or both eyes. Low blood pressure, nausea, loss of appetite, and vomiting from acute adrenal insufficiency. Nausea And Vomiting Paralysis Decreased Consciousness The most common symptoms include sudden severe headache with nausea and vomiting, double vision or loss of vision, change in mental status, loss of eye muscle control, and meningismus (symptoms associated with irritation of the brain and spinal cord). Other symptoms can include fever, pituitary failure, loss of consciousness, hypothalamic failure and death. Treatment It is usually treated by surgery soon after the symptoms occur. Pituitary apoplexy complications The hemorrhage- or necrosis-induced swelling can compress the: •Pituitary gland •Optic nerves •Nerves that control eye movement Pituitary apoplexy is a medical emergency and can be fatal if untreated. With treatment, however, the prognosis is good. Surgery is performed after medical stabilization |
Pheochromocytoma crisis
|
A pheochromocytoma is a rare, usually noncancerous (benign) tumor that develops in an adrenal gland. You have two adrenal glands —one located at the top of each kidney. Usually, a pheochromocytoma develops in only one adrenal gland. But tumors can develop in both.
If you have a pheochromocytoma, the tumor releases hormones that may cause high blood pressure, headache, sweating and symptoms of a panic attack. If a pheochromocytoma isn't treated, severe or life-threatening damage to other body systems can result. Most pheochromocytomas are discovered in people between the ages of 20 and 50. But the tumor can develop at any age. Surgery to remove a pheochromocytoma usually returns blood pressure to normal. Symptoms Signs and symptoms of pheochromocytomas often include: •High blood pressure •Headache •Heavy sweating •Rapid heartbeat •Tremors •Paleness in the face •Shortness of breath •Panic attack-type symptoms Less common signs or symptoms may include:v •Anxiety or sense of doom •Constipation •Weight loss Symptomatic spells The symptoms listed above may be constant, or they may occur, or get stronger, occasionally. Certain activities or conditions can make symptoms worse, such as: •Physical exertion •Anxiety or stress •Changes in body position •Labor and delivery •Surgery and anesthesia Foods high in tyramine, a substance that affects blood pressure, also can make symptoms worse. Tyramine is common in foods that are fermented, aged, pickled, cured, overripe or spoiled. These foods include: •Some cheeses •Some beers and wines •Chocolate •Dried or smoked meats Certain medications that can make symptoms worse include: •Monoamine oxidase inhibitors (MAOIs), such as phenelzine (Nardil), tranylcypromine (Parnate) and isocarboxazid (Marplan) •Stimulants, such as amphetamines or cocaine When to see a doctor Although high blood pressure is a primary sign of a pheochromocytoma, most people who have high blood pressure don't have an adrenal tumor. Talk to your doctor if any of the following factors are applicable to you: •Difficulty controlling high blood pressure with current treatment •Episodic worsening of high blood pressure •A family history of pheochromocytoma •A family history of a related genetic disorder: multiple endocrine neoplasia, type 2 (MEN 2); von Hippel-Lindau disease; familial paraganglioma or neurofibromatosis 1 (NF1) Causes Researchers don't know exactly what causes a pheochromocytoma. The tumor develops in specialized cells, called chromaffin cells, located in the center of an adrenal gland. These cells release certain hormones, primarily adrenaline (epinephrine) and noradrenaline (norepinephrine), that help control many body functions, such as heart rate, blood pressure and blood sugar. The role of hormones Adrenaline and noradrenaline trigger your body's fight-or-flight response to a perceived threat. The hormones cause your blood pressure to increase and your heart to beat faster. They prepare other body systems that enable you to react quickly. A pheochromocytoma causes more of these hormones to be released and causes them to be released when you're not in a threatening situation. Related tumors While most of the chromaffin cells are located in the adrenal glands, small clusters of these cells are also in the heart, head, neck, bladder, back wall of the abdomen and along the spine. Chromaffin cell tumors, called paragangliomas, may result in the same effects on the body. Risk factors Multiple endocrine neoplasia, type IIB (MEN IIB) People who have certain rare inherited disorders have an increased risk of pheochromocytoma or paraganglioma. Tumors associated with these disorders are more likely to be cancerous. These genetic conditions include: •Multiple endocrine neoplasia, type 2 (MEN 2) is a disorder that results in tumors in more than one part of the body's hormone-producing (endocrine) system. Other tumors associated with this condition can appear on the thyroid, parathyroid, lips, tongue and gastrointestinal tract. •Von Hippel-Lindau disease can result in tumors at multiple sites, including the central nervous system, endocrine system, pancreas and kidneys. •Neurofibromatosis 1 (NF1) results in multiple tumors in the skin (neurofibromas), pigmented skin spots and tumors of the optic nerve. •Hereditary paraganglioma syndromes are inherited disorders that result in either pheochromocytomas or paragangliomas. Complications High blood pressure can damage multiple organs, particularly tissues of the cardiovascular system, brain and kidneys. This damage can cause a number of critical conditions, including: •Heart disease •Stroke •Kidney failure •Problems with the nerves of the eye |
Acute hypercalcemia
|
What causes hypercalcemia? Which factors determine the severity of symptoms of hypercalcemia? What are the central nervous system (CNS) signs and symptoms of hypercalcemia? What are the renal signs and symptoms of hypercalcemia? What are the GI signs and symptoms of hypercalcemia? What are the cardiac signs and symptoms of hypercalcemia? How is hypercalcemia classified? How is the cause of hypercalcemia identified? What is the treatment of hypercalcemia? What is the pathophysiology of hypercalcemia? What are the intracellular and extracellular concentrations of calcium in the human body? What are the forms of calcium in plasma? Do intracellular stores of calcium have a role in plasma calcium homeostasis? What is a normal serum calcium level and how is hypercalcemia classified? What is the role of exchangeable calcium in the pathogenesis of hypercalcemia? What calculations or corrections are required to adjust for abnormal albumin levels in hypercalcemia? How are mild cases of hypercalcemia diagnosed? Which two mechanisms control calcium? What is the role of the calcium-sensing receptor (CaSR) in the pathogenesis of hypercalcemia? What is the etiology of hypercalcemia? What are the causes of hypercalcemia due to malignancy? What are the causes of hypercalcemia due to the parathyroid? What are the causes of hypercalcemia due to vitamin D? What are the causes of hypercalcemia due to high bone turnover? What are the less common causes of hypercalcemia? What is the prevalence of hypercalcemia by tumor type in the US? What is the global prevalence of hypercalcemia? What is the morbidity and mortality of hypercalcemia caused by hyperparathyroidism? What is the prevalence of hyperparathyroidism in patients with hypercalcemia? What is the role of PTH-related protein (PTHrP) in the etiology of hypercalcemia? What is the morbidity and mortality of hypercalcemia in patients with PTH-related protein (PTHrP) production or osteolytic metastases? How does the underlying cause of hypercalcemia affect morbidity and mortality? Are there sex-related variances in the incidence of hypercalcemia? Does the prevalence of hypercalcemia vary among age groups? What is the prognosis of tumor-related hypercalcemia? Does hypercalcemia increase mortality rates in patients with tumor? Presentation What are the signs and symptoms of hypercalcemia? What is the typical clinical presentation of hypercalcemia? What are the central nervous system (CNS) effects of hypercalcemia? What are the renal effects of hypercalcemia? What are the GI effects of hypercalcemia? What are the cardiac effects of hypercalcemia? Which physical findings suggest hypercalcemia? Which nervous system findings suggest hypercalcemia? Which renal findings suggest hypercalcemia? Which GI findings suggest hypercalcemia? Which cardiac findings suggest hypercalcemia? Which ophthalmic findings suggest hypercalcemia? DDX What are the differential diagnoses for Hypercalcemia? Workup What is the most common cause of hypercalcemia and when is it an unlikely cause? Which findings indicate tumor-caused hypercalcemia? What is the role of parathyroid hormone (PTH) testing in the diagnosis of hypercalcemia? Which lab tests are useful in determining the cause of hypercalcemia? What is the role of radiography in the diagnosis of hypercalcemia? What is the role of mammography, CT scanning, and ultrasonography in the diagnosis of hypercalcemia? Which imaging study is performed in the biochemical diagnosis of primary hyperparathyroidism in patients with hypercalcemia? What are the ECG characteristics of hypercalcemia? Treatment What is an effective short-term treatment for hypercalcemia? How is hypercalcemia treated once volume is restored? When is immobilization indicated in the management of hypercalcemia? What is the efficacy of dietary calcium and vitamin D reduction for the treatment of hypercalcemia? What is the role of bisphosphonates in the treatment of hypercalcemia? What is the role of denosumab (Xgeva) in the treatment of hypercalcemia? What is the role of dialysis in the treatment of patients with hypercalcemia? When is surgery indicated in the treatment of hypercalcemia? Which specialists may be consulted for patients with hypercalcemia? Medications What is the initial therapy for symptomatic hypercalcemia and what are the treatment options for more severe cases? Are bisphosphonates effective for treating hypercalcemia? Which medications in the drug class Calcimimetic Agent are used in the treatment of Hypercalcemia? Which medications in the drug class Minerals are used in the treatment of Hypercalcemia? Which medications in the drug class Glucocorticoids are used in the treatment of Hypercalcemia? Which medications in the drug class Antidote, hypercalcemia agents are used in the treatment of Hypercalcemia? Which medications in the drug class Monoclonal Antibodies, Endocrine are used in the treatment of Hypercalcemia? Which medications in the drug class Bisphosphonates are used in the treatment of Hypercalcemia? Here are further guidelines. |
Acute hypocalcaemia
|
What is hypocalcemia? What is the total calcium concentration in the plasma? How much calcium does each gram of albumin bind to at a plasma pH of 7.4? What is the role of calcium regulation in the pathogenesis of hypocalcemia? What is the role of ionized calcium in normal physiologic processes? How much calcium turnover occurs normally each day? What is the role of the parathyroid hormone (PTH), vitamin D, and calcitonin in the pathogenesis of hypocalcemia? How are ionized serum calcium changes recognized by the parathyroid gland in the pathophysiology of hypocalcemia? What is the role of calcium-sensing receptor (CaSR) in the pathophysiology of hypocalcemia? How is calcium homeostasis maintained? How is hypocalcemia defined? What are the triggers of tetany in patients with renal failure and hypocalcemia? What causes hypocalcemia? What is most common cause of hypocalcemia? What is the role of hypocalcemia in hyperphosphatemia? How is true hypocalcemia differentiated from factitious hypocalcemia? How is hereditary hypoparathyroidism differentiated from acquired hypoparathyroidism? What causes acquired hypoparathyroidism? What is polyglandular autoimmune disease (PGA I)? What causes hereditary hypoparathyroidism? Late-onset hypoparathyroidism is a symptom of which hereditary syndromes? What characterizes pseudohypoparathyroidism? How is pseudohypoparathyroidism classified? What is type Ia pseudohypoparathyroidism? What is type Ib pseudohypoparathyroidism? What is type Ic pseudohypoparathyroidism? What is type II pseudohypoparathyroidism? What is the etiology of hypomagnesemia? What causes vitamin D deficiency? What are the current Recommended Dietary Allowances (RDAs) for vitamin D? What is the effect of vitamin D deficiency in the elderly? Which conditions impair the absorption of vitamin D? What is pseudovitamin D deficiency rickets? What is the etiology of hereditary vitamin D resistance rickets? Which sources of vitamin D deficiency are caused by liver disease? When is calcidiol or calcitriol indicated for the treatment of hypocalcemia? What causes hypocalcemia in the early stages of renal failure? What is hungry bone syndrome? What is the role of hypocalcemia in pancreatitis? Do cinacalcet and chemotherapy drugs increase the risk of developing hypocalcemia? Can treatment of hypercalcemia with bisphosphonates cause hypocalcemia? Can prolonged use of anticonvulsants cause hypocalcemia? How does foscarnet cause hypocalcemia? What is denosumab? Can denosumab cause hypocalcemia? Can blood or plasma transfusion cause hypocalcemia? When might sodium phosphate bowel preparation cause acute hyperphosphatemia and subsequent hypocalcemia? How can radiographic contrast dyes cause hypocalcemia? Can an excess intake of fluoride cause hypocalcemia? Can proton pump inhibitors (PPIs) and histamine-2 receptor blockers (eg, cimetidine) cause hypocalcemia? Which medication effects may cause hypocalcemia? Can enhanced protein binding and anion chelation cause hypocalcemia? Which multifactorial causes are most clinically relevant in hypocalcemia emergencies? How does acute illness cause hypocalcemia? Can gram-negative sepsis cause hypocalcemia? Does sepsis increase the mortality rate for patients with hypocalcemia? Which surgical procedures may cause hypocalcemia? What is the incidence of hypocalcemia in the US? What are the risk factors for hypocalcemia? What are morbidity and mortality rates associated with hypocalcemia? What are the possible complications of chronic and severe hypocalcemia? Does the prevalence of hypocalcemia vary among age groups? Presentation What should be the focus of past medical history in the evaluation of hypocalcemia? Which neonates are at increased risk for developing hypocalcemia? How does hypocalcemia usually present? How is true hypocalcemia identified? Which disorders are associated with acute transient hypocalcemia? Which nutritional deficiency may cause hypocalcemia in elderly patients? Which drugs may cause hypocalcemia? Which environmental factors should be considered in the history for hypocalcemia? What are the neuromuscular symptoms of acute hypocalcemia? What are the neurologic symptoms of hypocalcemia? What are the dermatologic complications of chronic hypocalcemia? Which physical findings indicate hypocalcemia? Which head and neck exam findings indicate hypocalcemia? Which respiratory findings indicate hypocalcemia? What is the Chvostek sign in patients with hypocalcemia? What is the Trousseau sign in patients with hypocalcemia? Which movement abnormalities indicate hypocalcemia Which peripheral nervous system findings indicate hypocalcemia? DDX What is the first step in the evaluation of hypocalcemia? How are calcium levels corrected in patients with hypocalcemia and low serum albumin levels? Which gadolinium-based contrast agents interfere with the colorimetric assays for calcium testing? What are the differential diagnoses for Hypocalcemia? Workup How are most cases of hypocalcemia diagnosed? How is true hypocalcemia differentiated from factitious hypocalcemia? When are parathyroid hormone (PTH) and vitamin D testing indicated in the evaluation for hypocalcemia? Which imaging studies are indicated in the evaluation for hypocalcemia? What is the definitive method for diagnosing hypocalcemia? How should analysis for the ionized calcium level be performed in the diagnosis of hypocalcemia? Which factors cause falsely elevated or falsely depressed calcium levels in the evaluation for hypocalcemia? What stimulates phosphate excretion in patients with hypocalcemia? What is the usual presentation of patients with renal failure and hypocalcemia? Are serum magnesium levels measured in the evaluation for hypocalcemia? What is the role of parathyroid hormone (PTH) testing in the diagnosis of hypocalcemia? Which tests should be performed if vitamin D deficiency is the suspected cause of hypocalcemia? How is hypoparathyroidism differentiated from pseudohypoparathyroidism types I and II in patients with hypocalcemia? What is the role of electrocardiogram (ECG) in the diagnosis of hypocalcemia? Treatment What are the treatment options for hypocalcemia? What are the possible complications of severe hypocalcemia? Which medications are used to treat hypocalcemic emergencies? Should ICU patients with hypocalcemia receive calcium supplementation? What is the management for mild hypocalcemia? What supportive care should be given prior to direct treatment for severe hypocalcemia? What is the treatment for severe hypocalcemia? How is calcium delivered to patients with severe hypocalcemia? How often should serum calcium be measured in severe hypocalcemia? When is continuous ECG monitoring indicated in the treatment of severe hypocalcemia? How can severe hypocalcemia be prevented? What are the treatment options for chronic hypocalcemia? What is the treatment of severe hypoparathyroidism in patients with hypocalcemia? When is recombinant human parathyroid hormone (rhPTH, Natpara) indicated for treatment for chronic hypocalcemia? How can calcium levels be maintained in patients who have undergone parathyroidectomy? How is nutritional vitamin D and calcium deficiency treated in patients with hypocalcemia? Which dietary treatments are available for patients with chronic hypocalcemia? What information should patients with chronic hypocalcemia be given? Which specialist consultations may be necessary for hypocalcemia? How should treatment be directed after the cause of hypocalcemia has been determined? Medications What are the goals of pharmacotherapy for hypocalcemia? Which medications in the drug class Calcium are used in the treatment of Hypocalcemia? Which medications in the drug class Vitamin D are used in the treatment of Hypocalcemia? Which medications in the drug class Parathyroid Hormone Analogs are used in the treatment of Hypocalcemia? Here are further guidelines. |
Tests and Procedures
|
What is the range of normal human biochemistry laboratory values? Food and Drug Administration (FDA) 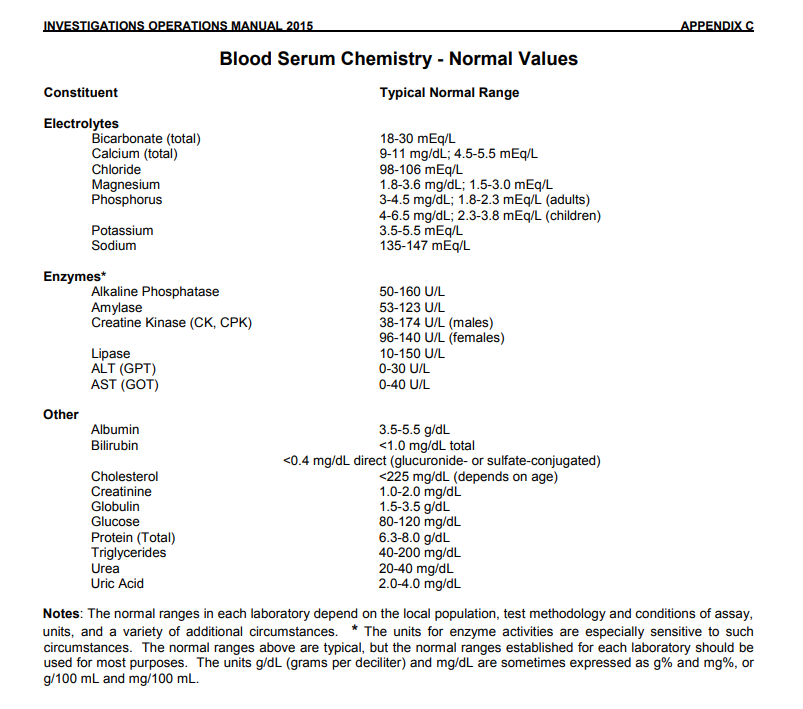 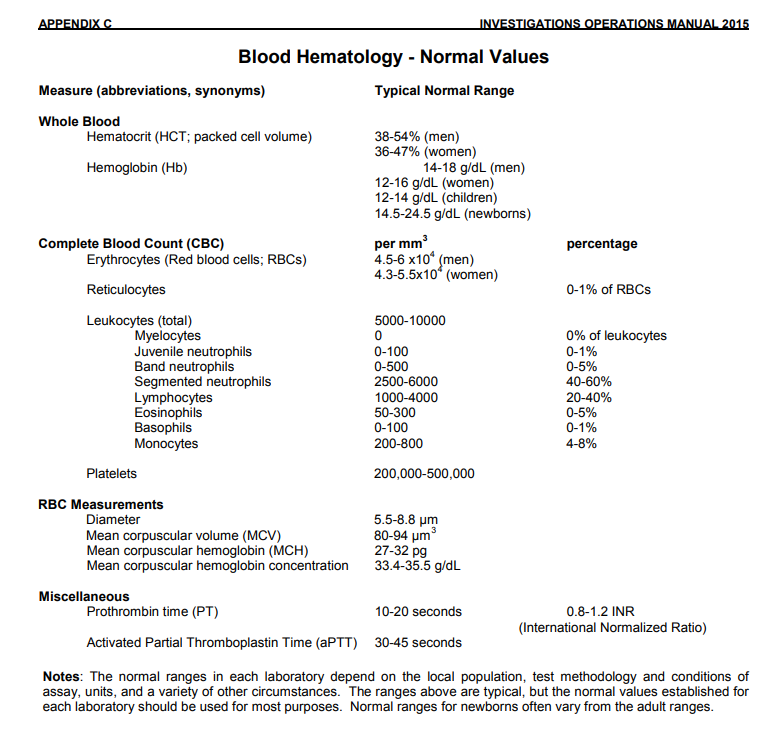 What ranges of normal human biochemistry values do you follow? What is your answer? |
Endocrine Emergencies
              |
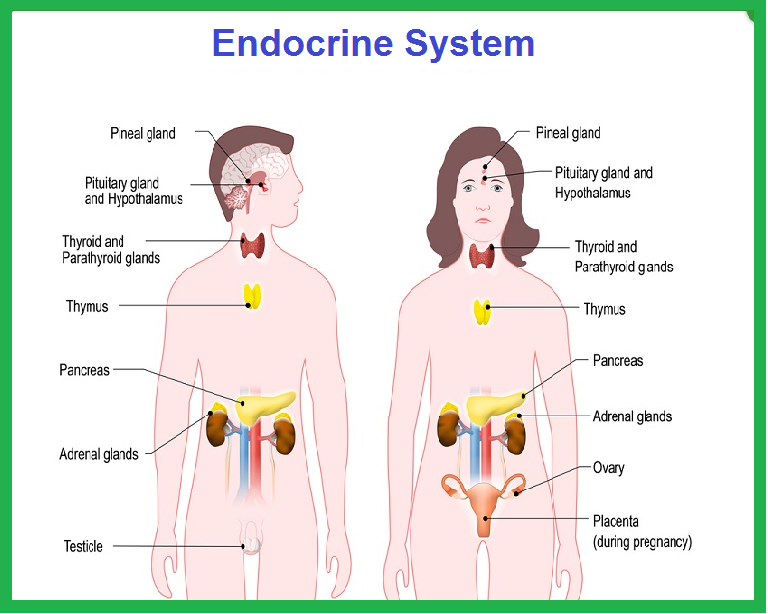
Hormones from Endocrine Glands
The glands of the endocrine system are:
Hypothalamus
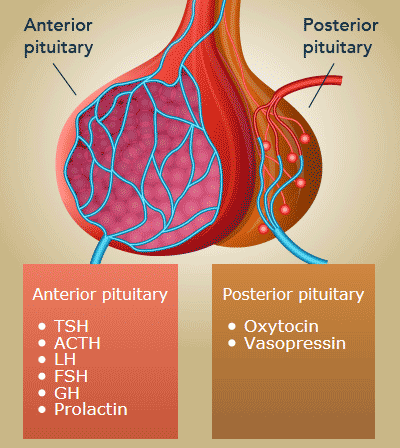
Pituitary GlandThe pituitary can be divided into the anterior and posterior gland. The hormones below are secreted by the anterior pituitary gland, except for ADH and oxytocin, which is secreted by the posterior pituitary gland.
Pineal GlandThe pineal gland was believed to play no significant role in the body an was thought of as a vestigial remnant. It is now known that the pineal gland secretes melatonin and related hormones which may play a role in sleep and possibly affect the secretion of luteinizing hormone and follicle-stimulating hormone. Thyroid
Parathyroid
Adrenal cortex
Adrenal medulla
Pancreas
Testes
Ovaries
Thymus GlandThe thymus gland in adults seems to have little functionality compared to early life but plays a role in the maturation of immune cells. It is not an endocrine gland. The following tissues and organs in the body also produce and secrete hormones but are not endocrine glands. Adipocytes (Fat Cells)
Heart
Kidney
Placenta
Stomach
Discussed under Digestive Hormones. Small intestine
Discussed under Digestive Hormones. |
Symptoms & Signs A-Z List
Diabetes Mellitus
Hyperthyroidism Hypothyroidism Myxedema Physical Findings Graves disease Acute Adrenal Insufficiency Clinical Presentation Pituitary Apoplexy Symptoms and Signs Pheochromocytoma Crisis Clinical Features Acute Hypercalcaemia Clinical Features Acute Hypocalcaemia Symptoms Signs Hypopituitarism: Symptoms & Signs The symptoms of hypopituitarism result from decreased hormone production by the pituitary gland. When all the pituitary hormones are affected, the condition is known as panhypopituitarism. Isolated or partial hypopituitarism results when the production of one or more hormones is decreased. The symptoms are variable and depend on the severity of the condition and the number of hormones that are affected. Symptoms can include |
Endocrine System
|
How many endocrine system organs are there in the human body? 9 Can you name the endocrine system organs in the human body?
Physiology of the Endocrine System Endocrinology
|
Endocrinologist
Endocrinology
|
What is an endocrinologist? What is endocrinology? What is the endocrine system? What do endocrinologists do? What is an endocrinologist? An endocrinologist is a specially trained clinician who is qualified to diagnosis conditions that affect the glands. They may diagnose and treat hormone imbalances in the endocrine organs, which include the pituitary, thyroid, adrenals, ovaries, testes and pancreas. What is endocrinology? Endocrinology is the study of hormones. Endocrinology is a branch of biology and medicine dealing with the endocrine system, its diseases, and its specific secretions known as hormones. What is the endocrine system? The endocrine system is a series of glands that produce and secrete hormones that the body uses for a wide range of functions. These control many different bodily functions, including: Respiration Metabolism Reproduction Sensory perception Movement Sexual development Growth Hormones are produced by glands and sent into the bloodstream to the various tissues in the body. They send signals to those tissues to tell them what they are supposed to do. When the glands do not produce the right amount of hormones, diseases develop that can affect many aspects of life. What specialists work with the issues relevant to the human endocrine system? Emergency medicine physician Critical care physician Primary care physician Endocrinologist br> Many primary care physicians, emergency medicine physicians, and critical care physicians are required relevant to these issues. Doctor Asif Qureshi is your guide. What do endocrinologists do? Conditions endocrinologists treat |
List of endocrine diseases
What are various examples?
|
Diabetes
| 1. Definition |
| 2. Causes |
| 3. Types |
| 4. Relevant anatomy, physiology, biochemistry |
| 5. Symptoms and signs |
| 6. Medical emergencies associated with this medical condition |
| 7. Risk factors |
| 8. Normal values |
| 9. Various diagnostic tests |
| 10. Diagnosis |
| 11. Complications |
| 12. Treatment or management |
| 13. Epidemiology |
| 14. History of this medical condition |
| 15. Prevention |
Diabetes mellitus
Type 1 Diabetes Type 2 Diabetes Gestational Diabetes Mature Onset Diabetes of the Young What is pre-diabetes? If a person is diagnosed with this, does it mean it will develop into diabetes? What is the difference between pre-diabetes and diabetes? What is the difference between type 1 and type 2 diabetes and their causes? What warning signs should a patient with a risk of diabetes look for? What are some tips for maintaining proper diet and exercise through the holidays? What foods or super foods should people with diabetes focus on during holiday gatherings? What is a healthy glucose level, and why is it important to maintain? Does eating a lot of sugar cause diabetes? Are artificial sweeteners safe? What’s the difference between carbohydrates and sugar? Is diabetes reversible? How is diabetes diagnosed? When is medication required? How long should medication last? What type of medication is available? Is diabetes reversible? How could this be prevented? Here are further guidelines. |
| Insulin |
| The thyroid gland |
| The adrenal gland |
| The parathyroid glands and vitamin D |
| The gonad |
| The pituitary gland |
| Cardiovascular and renal |
|
Public Alert Stay away from public affairs. |
This is in reference to a seminar on November 11, 2010, at SKIMS, Srinagar, Kashmir. The topic for discussion is Diabetes. I have written a book on diabetes. The state department of health can procure this book and circulate it to all relevant hospitals and departments. Women’s Health Signs & Symptoms Adolescence During adolescence, teenagers are always concerned about breast development, beginning menstrual cycles, and even shaving their legs. These may or may not be signs of endocrine disorders. What happens if a girl doesn't develop breasts or hair or have periods when expected? There is a wide variation in the time of expected puberty. If a girl has not developed breasts and axillary and pubic hair by the age of 14 and has not by age 16, she should undergo medical evaluation. Although in some cases, this may simply reflect either a family trait or a harmless deviation from "normal," this may be the first sign of a number of medical conditions, including thyroid, adrenal, pituitary or ovarian disorders. At times, undernutrition or excessive exercise may delay the onset of puberty (see below). Also, adrenal disease may be a consideration for girls who fail to begin menstruating or have irregular periods, excess facial and body hair, and acne. An early rapid growth spurt followed by a premature end to the growing period can cause short stature. Sometimes these symptoms can seem to have the same presentation as PCOS. Middle Age Type 2 diabetes Type 2 diabetes, hypertension and hypercholesterolemia are the most important and common risk factors for heart attacks in women. Women who have insulin resistance are at particular risk. Women with type 2 diabetes have an extremely high risk of heart disease and the symptoms of heart disease may be different from the typical symptoms. They may not have chest pain but merely fatigue, weakness during exercise, or other vague symptoms. Women with diabetes should be regularly tested for silent heart disease and should follow strict guidelines to control blood sugars, cholesterol, and blood pressure. Certain medications may be prescribed to reduce the risk of heart disease or reduce the risk of a second heart attack in a woman who has already had one. These may include statin drugs, aspirin, ACE inhibitors or Angiotensin blockers, and beta blockers. It is very important for women with diabetes to let their physicians know about any symptoms they might be having. |
Blood Sugar Level Ranges
|
Between 4.0 to 5.4 mmol/L (72 to 99 mg/dL) when fasting •Up to 7.8 mmol/L (140 mg/dL) 2 hours after eating |
| Target Levels by Type |
Upon waking | Before meals (pre prandial) |
At least 90 minutes after meals (post prandial) |
|---|---|---|---|
| Non-diabetic* | 4.0 to 5.9 mmol/L | under 7.8 mmol/L | |
| Type 2 diabetes | 4 to 7 mmol/L | under 8.5 mmol/L | |
| Type 1 diabetes | 5 to 7 mmol/L | 4 to 7 mmol/L | 5 to 9 mmol/L |
| Children w/ type 1 diabetes | 4 to 7 mmol/L | 4 to 7 mmol/L | 5 to 9 mmol/L |
|
*The non-diabetic figures are provided for information but are not part of NICE guidelines. Normal and diabetic blood sugar rangesFor the majority of healthy individuals, normal blood sugar levels are as follows:
For people with diabetes, blood sugar level targets are as follows:
Blood sugar levels in diagnosing diabetesThe following table lays out criteria for diagnoses of diabetes and prediabetes.
Random plasma glucose testA blood sample for a random plasma glucose test can be taken at any time. This doesn’t require as much planning and is therefore used in the diagnosis of type 1 diabetes when time is of the essence. Fasting plasma glucose testA fasting plasma glucose test is taken after at least eight hours of fasting and is therefore usually taken in the morning. The _____ guidelines regard a fasting plasma glucose result of 5.5 to 6.9 mmol/l as putting someone at higher risk of developing type 2 diabetes, particularly when accompanied by other risk factors for type 2 diabetes. Oral Glucose Tolerance Test (OGTT)An oral glucose tolerance test involves taking a first taking a fasting sample of blood and then taking a very sweet drink containing 75g of glucose. After having this drink you need to stay at rest until a further blood sample is taken after 2 hours. HbA1c test for diabetes diagnosisAn HbA1c test does not directly measure the level of blood glucose, however, the result of the test is influenced by how high or low your blood glucose levels have tended to be over a period of 2 to 3 months. Indications of diabetes or prediabetes are given under the following conditions:
|
List of Endocrine Disorders / Most common and least common
Prevention
|
How can you prevent endocrine problems? Can endocrine system diseases be prevented? “Endocrine disease” encompasses many medical problems, so there isn’t a simple yes or no answer to this question. Certainly in the case of diabetes, one of the most common endocrine problems, there is a great deal that one can do to prevent disease. Maintaining a normal body weight, consuming a diet low in highly-processed carbohydrates and exercising regularly can all reduce the risk of diabetes. On the other hand, most endocrine disorders are not the result of lifestyle issues. Endocrine disease often means under-production of a hormone. Examples here are hypothyroidism, adrenal insufficiency and premature ovarian failure (sometimes called premature or early menopause) or male hypogonadism (testosterone deficiency). In many cases these are the result of an autoimmune attack against the hormone-producing gland. There are usually no environmental or lifestyle issues that influence these conditions. Tumors, infections, surgery or trauma may also cause under-production of hormones. Tumors may also cause hormone over-production, or may cause symptoms by virtue of their size even without affecting hormone production. However, these are not the result of lifestyle issues and generally are not preventable. The basis of health care is that it is much better for the individual member of society to be healthy and well than to be ill or deceased. To assist the individual in staying alive and well the health care system provides a broad range of services aimed at cure or control of disease. These services are available when someone becomes ill. A different approach to preservation of good health and longevity is the prevention of disease. Prevention may take many forms. This may vary from legislation on food declaration via public campaigns on the importance of physical exercise to neonatal screening programmes. The intervention may be directed at decreasing the risk for disease in healthy subjects (primary prevention). A common variation is prevention of the severe consequences of disease by early detection of subclinical disease by screening or case-finding (secondary prevention). Other variants are prevention of complications of disease (tertiary prevention) or prevention of recurrence of disease by secondary intervention. |